
10 Best Clinical Trial Management Software for 2025 & Beyond
As the healthcare landscape continues to evolve, clinical data management software plays an increasingly vital role in enhancing the efficiency of clinical trials and improving overall patient care. In 2025, a range of innovative software solutions will redefine how Clinical Research Organizations (CROs) and pharmaceutical companies manage their research operations.
A Clinical Trial Management System (CTMS) is essential for conducting clinical studies involving human subjects. These CTMS platforms are widely used by pharmaceutical companies, hospitals, universities, and private research firms to ensure seamless trial execution, compliance, and data accuracy.
Below, we’ve compiled a list of the 20 best clinical trial management systems designed to streamline operations, accelerate research, and enhance decision-making in clinical trials.
What is clinical trial management software?
A Clinical Trial Management System (CTMS) is an integrated software solution designed to streamline the planning, tracking, and management of clinical trials. These systems centralize data, automate workflows, and ensure compliance with global regulatory standards, helping research organizations efficiently conduct trials.
CTMS platforms provide essential functionalities such as trial planning, benchmarking, and reporting, ensuring seamless execution. Additionally, an effective CTMS should either include built-in business intelligence and analytics features or support integration with third-party analytics platforms to enhance data-driven decision-making.
Key Clinical Trial Management Software Statistics
Clinical trial sponsors have long looked for creative ways to use technology breakthroughs to make clinical studies quicker, more effective, and more patient-friendly. By drastically altering the clinical trial environment and patient experience, the COVID-19 pandemic in 2020 greatly expedited the deployment of decentralized clinical trial (DCT) components. The numbers that follow demonstrate the increasing influence of contemporary technology on:
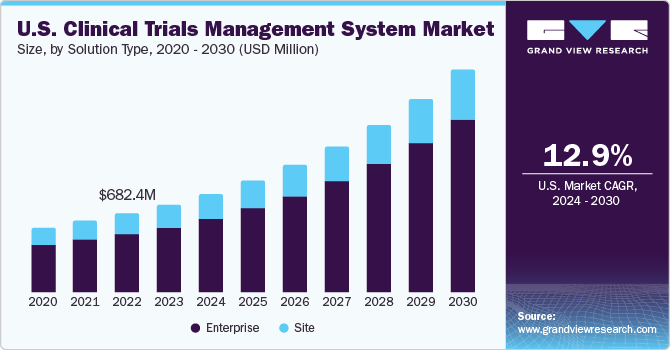
- The global clinical trials management system market size was estimated at USD 1.85 billion in 2023 and is projected to grow at a CAGR of 14.65% from 2024 to 2030.
- A new Clinical Trial Landscape program was introduced in 2024 by Research Solution, a top supplier of cloud-based workflow solutions, to improve the usability, actionability, and insights obtained from clinical trial data.
- 57% of clinical trial investigators conducted virtual patient interactions during the peak of COVID-19, compared to just 9% before the pandemic (Agrawal et al., 2021).
- 77% of U.S.-based investigators anticipate a rise in remote patient monitoring procedures post-pandemic.
List of Top 10 Clinical Trial Management Software for 2025
Here’s a list of some of the best Clinical Trial Management Software (CTMS) for 2025, based on market presence, features, and user reviews:
Labfront: A Comprehensive Clinical Trial Management Platform
Labfront is an innovative electronic data capture (EDC) software designed to streamline data collection for research, clinical trials, and studies. This healthcare management platform provides top researchers from private research companies with advanced tools to create and manage electronic case report forms (eCRFs), ensuring accurate, efficient, and compliant data capture.
Key aspects of Labfront CTMS:
- Customizable Data Collection Protocols: Tailor data collection to meet specific research needs.
- Participant Management: Efficiently track and manage participant information for better oversight.
- Regulatory Compliance: Built-in security and validation features to meet global regulatory standards.
EDGE: The Program Designed for Fast and Accurate Data Sharing
EDGE is a powerful Clinical Trial Management System (CTMS) designed to streamline data sharing, enhance collaboration, and improve research efficiency. This cloud-based platform is widely used by hospitals, research institutions, and pharmaceutical companies to manage clinical trials with greater speed and accuracy.
Key aspects of EDGE CTM software:
- Real-Time Data Sharing: Facilitates instant access to trial data for research teams.
- Workflow Optimization: Automates administrative tasks to enhance efficiency.
- Regulatory Compliance: Ensures adherence to global clinical trial regulations.
- Customizable Dashboards: Provides insights through analytics and reporting tools.
- Secure Cloud-Based Access: Enables seamless collaboration across multiple locations.
Viedoc: A Next-Generation Cloud-Based Solution for Streamlining Clinical Trials
The Viedoc eClinical suite is a preferred choice for pharmaceutical, biotech, and medical device companies and research institutions worldwide. Designed to enhance the efficiency of clinical trials, Viedoc makes every aspect of a study more intuitive, user-friendly, and streamlined, while its SaaS platform ensures the technology is more reliable, scalable, and cost-effective.
Key aspects of Viedoc CTMS:
- Cloud-Based SaaS Platform: Ensures high scalability, reliability, and cost-effectiveness.
- Intuitive User Experience: Simplifies trial management with a user-friendly interface.
- Regulatory Compliance: Meets FDA, EMA, GCP, and GDPR standards for global trials.
- Comprehensive eClinical Suite: Supports EDC, ePRO, eTMF, CTMS, and analytics.
- Automation & AI Integration: Enhances workflow efficiency and data accuracy.
- Seamless System Integration: Connects with third-party tools for a unified research ecosystem.
Dot Compliance: Ensuring Regulatory Compliance in Clinical Trial Operation
Dot Compliance is a comprehensive quality management system (QMS) designed to support clinical trial operations by ensuring regulatory compliance, risk management, and streamlined documentation. Global compliance standards such as FDA, EMA, ISO, and GxP are fulfilled by pharmaceutical, biotech, and medical device companies with the use of this cloud-based application.
Key aspects of Dot Compliance:
- Automated Compliance Management: Ensures adherence to regulatory requirements with built-in compliance tracking.
- Document Control & Audit Readiness: Centralized storage and version control for SOPs, trial protocols, and study reports.
- Risk Management & CAPA: Identifies, assesses, and mitigates risks while automating Corrective and Preventive Actions (CAPA).
- Seamless Integration: Connects with existing CTMS, EDC, and eTMF systems for a unified workflow.
- Collaboration: Improves collaboration by providing a centralized platform for clinical teams.
OpenClinica: An Advanced Open-Source CTMS for Efficient Trial Management
Since 2006, OpenClinica has been a pioneer in delivering innovative and effective clinical trial solutions. By automating electronic data capture (EDC) with dynamic, fully customizable electronic case report forms (eCRFs), OpenClinica enables researchers to collect high-quality data faster and more efficiently. 3+ million patients have participated in OpenClinica-powered trials. It is trusted by leading life sciences companies, academic institutions, and government agencies across the globe.
Key aspects of the OpenClinica platform:
- Advanced EDC System: Automates data capture, validation, and processing.
- Custom eCRFsL: Enables researchers to design flexible, study-specific data collection forms.
- Regulatory Compliance: Ensures adherence to FDA 21 CFR Part 11, HIPAA, and ICH-GCP.
- Real-Time Analytics & Reporting: Delivers actionable insights through dashboards and visualizations.
- Cloud-Based & Scalable: Supports multi-site, global trials with high security.
QuesGen Platform: A Specialized Solution for Managing Clinical Research Data
QuesGen CTMS System provides services extending from data management to comprehensive CRO services. Having grown up in academic research, QuesGen now also works with industry. Their web-based, adaptable software solution offers a customizable and user-friendly CRF (Case Report Form) environment. You can utilize and modify our database as necessary thanks to the platform, which is a complete solution made especially for maintaining and setting clinical datasets.
Key aspects of the QuesGen Platform:
- Regulatory Compliance: Meets HIPAA, FDA 21 CFR Part 11, and GCP guidelines for secure research data management.
- Customizable Study Design: Offers flexible form creation, automated workflows, and tailored study configurations.
- Integrated Analytics & Reporting: Provides real-time insights, visualization tools, and data exports.
- Cloud-Based & Scalable: Supports multi-site trials with high data security and accessibility.
- Advanced Security Measures: Ensures encrypted data storage, role-based access, and audit trails for compliance.
Climedo: A Decentralized Clinical Trial Platform for Real-World Evidence Collection
Climedo is an innovative electronic Clinical Outcomes Assessment (eCOA) and Electronic Data Capture (EDC) solution designed to support real-world evidence (RWE) collection and non-interventional research (NIS). This hybrid clinical trial platform enhances collaboration between clients, key opinion leaders (KOLs), and medical professionals, ensuring efficient trial execution and regulatory compliance.
Key aspects of the Climedo COA solution:
- Trusted by Over 1.7 Million Patients & Leading CROs: A proven partner for academics, medtech firms, pharmaceutical companies, and contract research organizations (CROs).
- Scalable & Future-Ready: This clinic management software is designed for small-scale clinical studies as well as large multi-center global trials.
- Data-Driven Decision Making: AI-powered analytics provide actionable insights for faster regulatory approvals and better patient outcomes.
- User-Friendly & Mobile-Optimized: An intuitive interface ensures easy adoption for patients, investigators, and site coordinators.
hCue Clinical Management Software: Simplifying Clinical Trial Administration and Data Handling
hCue is a leading cloud-based Clinic Management Software (CMS) designed to simplify clinic operations for doctors, healthcare providers, and medical institutions. This all-in-one clinical trial platform enhances patient management, appointment scheduling, and collaboration with pharmacies and labs, ensuring seamless healthcare delivery. It is a proven clinical management software for general practitioners, specialists, and multi-chain healthcare providers in India.
Key Features of the hCue CMS:
- Smart Appointment Scheduling: This enables doctors to manage patient visits efficiently with online booking, automated reminders, and real-time availability tracking.
- Seamless Integration with Pharmacies & Labs: Connects healthcare providers with diagnostic labs and pharmacies, facilitating e-prescriptions and test referrals.
- Enhanced Doctor Discovery: Makes practitioners more accessible to potential patients through search engine optimization and online presence management.
- Collaborative Referral System: Supports doctor-to-doctor referrals, enabling specialists to refer patients and receive referrals within the hCue network.
- Cloud-Based & Mobile-Optimized: Ensures 24/7 accessibility from any device, providing a hassle-free paperless clinic experience.
Track.Health: A Patient-Centric Platform for Remote Monitoring and Trial Management
The term “Track.Health” describes a healthcare digital platform used by healthcare management companies that put the patient experience first by facilitating remote monitoring of medical data and clinical trial management. This allows patients to take an active role in their healthcare journey and gives medical professionals real-time information about their condition from a convenient, easily accessible location.
Key aspects of Track.Health:
- Telemedicine & Virtual Visits: Facilitates secure video consultations between patients and trial investigators, reducing the need for in-person visits.
- Interoperability & Data Integration: Seamlessly integrates with EHRs, wearable health devices, and third-party CTMS platforms, enhancing data accuracy and accessibility.
- Enhanced Patient Engagement & Retention: A user-friendly interface and real-time health tracking improve patient compliance.
- Regulatory Compliance & Data Security: Adheres to HIPAA, GDPR, and FDA guidelines to ensure secure, privacy-focused clinical trials.
- Scalable for Global Clinical Trials: Supports multi-center trials, enabling researchers to conduct studies across different geographies efficiently.
Sofpromed eCRF: A Secure Electronic Data Capture System for Clinical Trials
Sofpromed eCRF is a highly secure and compliant electronic data capture (EDC) system designed to streamline clinical trial data management. It is tailored for pharmaceutical companies, Contract Research Organizations (CROs), and research institutions. This healthcare tracking platform is designed to optimize workflows, increase efficiency, and improve data accuracy in clinical trials, making it a valuable tool for healthcare organizations involved in medical research.
Key aspects of Sofpromed eCRF:
- Streamlining Data Management: Efficiently collecting, validating, and analyzing trial data.
- Ensuring Compliance: Maintaining regulatory standards to avoid compliance issues.
- Improving Data Accuracy: Reducing errors through real-time validation and automated checks.
- Enhancing Collaboration: Integrating with existing systems to foster a collaborative environment.
What Are the Most Important Considerations for Clinical Trial Asset Tracking Implementation?
When implementing asset tracking in clinical trials, Healthcare management software development companies should consider several key factors to ensure effective management and compliance. Here are some of the main considerations:
- Scalability: Ensure the asset tracking system can adapt to future growth and changes in asset inventories. This includes the ability to handle increased data volume and expand to new locations if necessary.
- Integration: Evaluate compatibility with existing systems to facilitate seamless integration and data exchange. This minimizes disruption and maximizes the value of interconnected technologies.
- Data Security: Implement robust security measures to safeguard sensitive asset data from unauthorized access, breaches, or cyber threats. Ensure confidentiality, integrity, and availability of critical information.
- Regulatory Compliance: Adhere to relevant data privacy regulations, industry standards, and best practices for asset management. This mitigates legal and reputational risks while fostering trust and credibility within the industry.
- Asset Identification and Tracking: Assign unique identifiers to each asset and track movements and locations through a database. This can include information such as purchasing date, cost, current location, and maintenance records.
- Technology Selection: Choose appropriate tracking technologies based on the type of assets and environment. Options include RFID, GPS, LoRaWAN, Wi-Fi, or cellular networks, each with its advantages in terms of range, power consumption, and security.
- Cost and ROI: Assess the costs associated with implementing and maintaining the asset tracking system. Evaluate the return on investment (ROI) by considering factors like reduced asset loss, improved efficiency, and enhanced patient care.
What is The Cost of Hiring CTMS Developers?
The cost of Clinical Trial Management Systems (CTMS) can vary widely based on several factors, including the size of the organization, the features required, and the vendor chosen. Make sure to hire the best CTMS developers from reputable app development companies to ensure the correct implementation of all the factors. Here’s a general breakdown:
Licensing Models:
- Subscription-Based: Many CTMS solutions operate on a subscription model, which can range from $1,000 to $10,000 per month, depending on the number of users and features.
- Per-Study Licensing: Some vendors charge based on the number of studies being managed, which could cost anywhere from $5,000 to $50,000 per study.
Implementation Costs:
Initial setup and implementation can add significant costs, typically ranging from $10,000 to $100,000, depending on the complexity of the system and the level of customization required.
Additional Costs:
- Training: Training staff on the new system may incur additional costs, often ranging from $1,000 to $10,000.
- Support and Maintenance: Ongoing support and maintenance can also add to the total cost, often charged as a percentage of the licensing fee.
Total Cost of Ownership:
- Over a multi-year period, the total cost of CTMS ownership can range from $50,000 to several million dollars, depending on the factors mentioned above.
- When considering a CTMS, it’s essential to evaluate both the upfront and ongoing costs, as well as the specific needs of your organization to find the best fit.
By providing these services, HiTechHub can help organizations navigate the complex process of selecting and implementing a CTMS, ensuring a successful integration that enhances clinical trial efficiency and compliance.
How HiTech Hub Supports CTMS Selection and Implementation
HiTech Hub stands out as a comprehensive platform for those seeking reliable and up-to-date information on technology advancements and service providers in the digital landscape. Our skilled profession assists organizations in choosing and implementing Clinical Trial Management Software (CTMS) by providing expert insights, curated rankings, and strategic recommendations. Here’s how we can help:
Research & Industry Insights
HiTech Hub delivers in-depth articles, industry trends, and research reports on clinical trial management, regulatory compliance, and CTMS advancements. This helps businesses, researchers, and healthcare professionals stay updated on the latest tools and best practices.
Curated Lists of Top CTMS Providers
HiTech Hub compiles rankings of leading CTMS solutions, evaluating them based on:
- Data security & compliance (HIPAA, GDPR, 21 CFR Part 11)
- Ease of use & scalability
- Integration with EDC, ePRO, and eCOA systems
- Customer reviews & success stories
Comparative Analysis & Expert Recommendations
For organizations looking to adopt or upgrade a CTMS, HiTech Hub provides side-by-side comparison, a list of top competitors, and industry giants offering clinical trial management software solutions, helping them identify solutions that align with their specific needs—whether in pharmaceuticals, biotech, or academic research.
Connecting Businesses with CTMS Providers
HiTech Hub serves as a bridge between software vendors and potential clients, offering:
- Direct links to service providers
- Comprehensive reviews and ratings
- Contact options for seamless communication
Software Development & Customization
For businesses needing custom CTMS solutions, HiTech Hub recommends top Android mobile app development companies, software developers, and IT service providers specializing in tailored clinical trial platforms.
Support for CTMS Implementation
- Implementation Planning: HiTech Hub can aid in creating a detailed implementation plan, ensuring alignment with organizational timelines and milestones.
- Team Involvement and Training: They can facilitate team involvement from the outset, ensuring that users are engaged in the selection process and trained on the new system to maximize adoption and efficiency.
- Integration and Data Migration: HiTech Hub can support the integration of the CTMS with existing systems (e.g., EDC, eTMF) and manage data migration to ensure seamless transition and minimal disruption.
- Compliance and Security: They can verify that the CTMS meets all necessary regulatory requirements and implement robust security measures to protect sensitive data
By providing expert guidance and trusted resources, HiTech Hub simplifies the CTMS selection and implementation process, enabling organizations to streamline clinical trial operations with efficiency and confidence.
Key Takeaway!
As clinical research continues to evolve, adopting advanced CTMS and EDC solutions is essential for ensuring accurate, compliant, and efficient trial management. These platforms go beyond data collection and monitoring—they enhance collaboration, improve patient engagement, and accelerate trial timelines, ultimately driving more effective and successful clinical research outcomes.
With HiTechHub’s expertise, businesses and researchers can make informed decisions, optimize trial processes, and stay ahead in the rapidly advancing clinical research landscape. Whether you’re looking for the best CTMS providers, comparative analysis, or custom software development, HiTechHub is your trusted partner in navigating the future of clinical trial management.
FAQs
What is a Clinical Trial Management System (CTMS)?
A CTMS is a software platform designed to manage, track, and streamline clinical trial operations, including patient enrollment, data collection, compliance, and reporting. It helps research organizations enhance efficiency, improve collaboration, and reduce errors in trial management.
Why is CTMS important for clinical trials?
CTMS is essential for:
- Centralizing trial data for better management
- Ensuring regulatory compliance (FDA, EMA, HIPAA, etc.)
- Automating workflows to reduce manual effort
- Enhancing patient engagement with remote monitoring
- Improving decision-making with real-time analytics
How do these CTMS solutions enhance clinical trial management?
These CTMS platforms help research organizations by:
- Reducing trial costs and timelines through automation
- Improving data integrity with real-time tracking and validation
- Facilitating collaboration between research teams and stakeholders
- Enhancing patient recruitment and retention.
What key features should I look for in a CTMS?
When choosing a CTMS, look for:
- Electronic Data Capture (EDC) and eCRF integration
- Automated workflows and real-time monitoring
- Regulatory compliance management
- Business intelligence and analytics tools
- Remote patient monitoring and decentralized trial support
Eshika Jain
25-February-2025




